The first and only relatively non-selective alpha-1 and alpha-2 adrenergic antagonist to reverse pharmacologically-induced mydriasis.1
How RYZUMVI® works1,2:
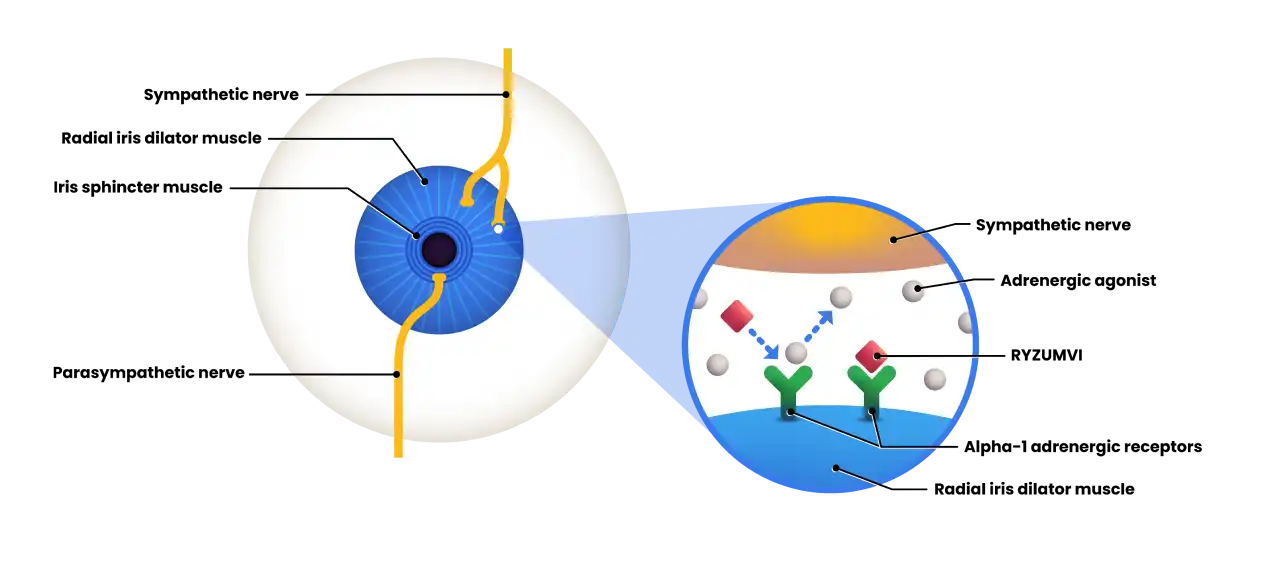
RYZUMVI reversibly binds to alpha-1 adrenergic receptors on the radial iris dilator muscle and indirectly reverses the effects of muscarinic antagonists on the iris sphincter muscle, thus reducing pupil size after dilation.1